Agonists of the phencyclidine
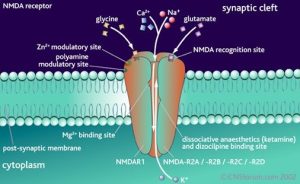
There are a number of non-competitive NMDA receptor inhibitors acting on the phencyclidine site located in the ion channel, which act on receptors only in the presence of agonists. It follows that the mechanism of their action is to block the open ion channel.
Phencyclidine is a highly affinity NMDA receptor antagonist. Modification of the phencyclidine molecule by introducing new aromatic cycles led to the creation of highly affinity antagonists of the phencyclidine site.
1‑aminoadamantane derivatives are an important structural class of compounds exhibiting antagonistic activity against the phencyclidine site of NMDA receptors.
The introduction of small alkyl substituents at positions 3 and 5 enhances antagonistic activity, while the substitution of at least one hydrogen at the nitrogen atom leads to its sharp decrease.
One of the most striking compounds of this type is memantine, which is used to treat neurodegenerative diseases. The absence of an endogenous agonist with respect to the phencyclidine site does not allow us to unambiguously link together all the structural groups of its antagonists that differ greatly from each other, therefore, attempts are currently being made to create complex models of the phencyclidine site.
Ionotropic glutamate receptors ampa – kainate subtype
AMPA is a subtype of glutamate receptors, of which AMPA is a selective agonist. They contain both a site binding to competing agonists and antagonists (glutamate) and interaction sites with non-competitive (allosteric) inhibitors.
The kainate subtype of glutamate receptors, the selective agonist of which is kainic acid.
Agonists
The AMPA molecule is an analog of glutamic acid, in which the role of the terminal carboxyl group is played by the acidic hydroxyisoxazole group. Only the S‑isomer of AMPA is active to the corresponding receptor, while the R‑isomer is practically inactive. The replacement of hydrogen atoms of the methyl group with a halogen (-CF3, -CH2Cl) leads to agonists with activity close to that of AMPA. However, the introduction of a volumetric tretbutyl group leads to a loss of agonistic activity against the kainate receptor. The replacement of the hydroxyl group in isoxazole with a carboxyl group leads to a fivefold increase in activity as an antagonist.
Natural β‑oxosylamino‑L‑alanine is also a strong agonist of AMPA.
The structures of currently known kain receptor agonists are quite similar to kainic acid itself. An interesting example of the construction of an AMPA kainate receptor agonist is the combination of an AMPA fragment and kainic acid in one molecule.
Proline derivatives have weak agonistic activity against the AMPA receptor, but are also capable of binding to the AMDA receptor.
The proline isomer is a highly affine and active agonist of AMPA kainate receptors, although, as one might expect, it is not too selective with respect to each of the subtypes. A very strong and very selective ligand of the kainate receptor is such a simple compound as 4‑methylglutamic acid, which is 3,000 times more selective to the kainate than to the AMPA receptor and 200 times more selective to the kainate receptor than the NMDA receptor.
The structural requirements for the selection of selective agonists of the glutamic acid binding site of AMPA kainate receptors, which distinguish them from those for NMDA receptors, are not completely clear, except for the preference of the S‑configuration of the chiral amino acid center.
